Enzymes are used in Starch Hydrolysis
Starch is the
commonest storage carbohydrate in plants. It is used by the plants themselves,
by microbes and by higher organisms so there is a great diversity of enzymes
able to catalyse its hydrolysis. Starch from all plant sources occurs in the
form of granules which differ markedly in size and physical characteristics from
species to species. Chemical differences are less marked. The major difference
is the ratio of amylose to amylopectin; e.g. corn starch from waxy maize
contains only 2% amylose but that from amylomaize is about 80% amylose. Some
starches, for instance from potato, contain covalently bound phosphate in small
amounts (0.2% approximately), which has significant effects on the physical
properties of the starch but does not interfere with its hydrolysis. Acid
hydrolysis of starch has had widespread use in the past. It is now largely
replaced by enzymic processes, as it required the use of corrosion resistant
materials, gave rise to high colour and saltash content (after neutralisation),
needed more energy for heating and was relatively difficult to control.

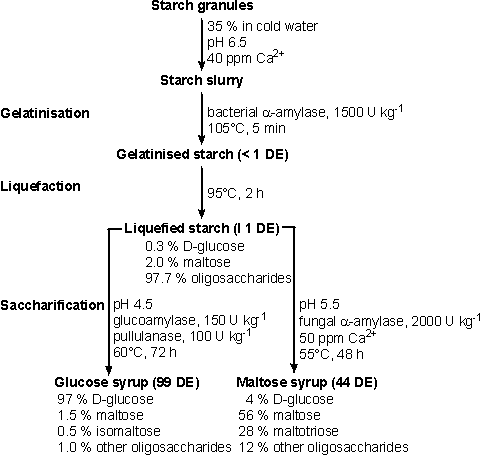
Figure 1. The
use of enzymes in processing starch. Typical conditions are given.
Of the two
components of starch, amylopectin presents the great challenge to hydrolytic
enzyme systems. This is due to the residues involved in a-1,6-glycosidic branch
points which constitute about 4 - 6% of the glucose present. Most hydrolytic
enzymes are specific for a-1,4-glucosidic links yet the a-1,6-glucosidic links
must also be cleaved for complete hydrolysis of amylopectin to glucose. Some of
the most impressive recent exercises in the development of new enzymes have
concerned debranching enzymes.
It is
necessary to hydrolyse starch in a wide variety of processes which m be
condensed into two basic classes:
In the former
processes, such as glucose syrup production, starch is usually the major
component of reaction mixtures, whereas in the latter processes, such as the
processing of sugar cane juice, small amounts of starch which contaminate non-starchy
materials are removed. Enzymes of various types are used in these processes.
Although starches from diverse plants may be utilised, corn is the world's most
abundant source and provides most of the substrate used in the preparation of
starch hydrolysates.
There are
three stages in the conversion of starch (Figure 1):
Gelatinisation
is achieved by heating starch with water, and occurs necessarily and naturally
when starchy foods are cooked. Gelatinised starch is readily liquefied by
partial hydrolysis with enzymes or acids and saccharified by further acidic or
enzymic hydrolysis.
The starch
and glucose syrup industry uses the expression dextrose equivalent or DE,
similar in definition to the DH units of proteolysis, to describe its products,
where:
![]() (1.1)
(1.1)
In practice,
this is usually determined analytically by use of the closely related, but not
identical, expression:
![]() (1.2)
(1.2)
Thus, DE
represents the percentage hydrolysis of the glycosidic linkages present. Pure
glucose has a DE of 100, pure maltose has a DE of about 50 (depending upon the
analytical methods used; see equation (1.2)) and starch has a DE of effectively
zero. During starch hydrolysis, DE indicates the extent to which the starch has
been cleaved. Acid hydrolysis of starch has long been used to produce 'glucose
syrups' and even crystalline glucose (dextrose monohydrate). Very considerable
amounts of 42 DE syrups are produced using acid and are used in many
applications in confectionery. Further hydrolysis using acid is not satisfactory
because of undesirably coloured and flavoured breakdown products. Acid
hydrolysis appears to be a totally random process which is not influenced by the
presence of a-1,6-glucosidic linkages.
Table 2.Enzymes
used in starch hydrolysis
| Enzyme | EC number | Source |
Action |
| a-Amylase | 3.2.1.1 | Bacillus amyloliquefaciens | Only a-1,4-oligosaccharide links are cleaved to give a-dextrins and predominantly maltose (G2), G3, G6 and G7 oligosaccharides |
| B. licheniformis | Only a-1,4-oligosaccharide links are cleaved to give a-dextrins and predominantly maltose, G3, G4 and G5 oligosaccharides | ||
| Aspergillus oryzae, A. niger | Only a-1,4 oligosaccharide links are cleaved to give a-dextrins and predominantly maltose and G3 oligosaccharides | ||
| Saccharifying a-amylase | 3.2.1.1 | B. subtilis (amylosacchariticus) | Only a-1,4-oligosaccharide links are cleaved to give a-dextrins with maltose, G3, G4 and up to 50% (w/w) glucose |
| b-Amylase | 3.2.1.2 | Malted barley | Only a-1,4-links are cleaved, from non-reducing ends, to give limit dextrins and b-maltose |
| Glucoamylase | 3.2.1.3 | A. niger | a-1,4 and a-1,6-links are cleaved, from the nonreducing ends, to give b-glucose |
| Pullulanase | 3.2.1.41 | B. acidopullulyticus | Only a-1,6-links are cleaved to give straight-chain maltodextrins |
The
nomenclature of the enzymes used commercially for starch hydrolysis is somewhat
confusing and the EC numbers sometimes lump together enzymes with subtly
different activities (Table 2)For example, a-amylase may be subclassified as
liquefying or saccharifying amylases but even this classification is inadequate
to encompass all the enzymes that are used in commercial starch hydrolysis. One
reason for the confusion in the nomenclature is the use of the anomeric form of
the released reducing group in the product rather than that of the bond being
hydrolysed; the products of bacterial and fungal a-amylases are in the a-configuration
and the products of b-amylases are in the b-configuration, although all these
enzymes cleave between a-1,4-linked glucose residues.
The a-amylases
(1,4-a-D-glucan glucanohydrolases) are endohydrolases which cleave 1,4-a-D-glucosidic
bonds and can bypass but cannot hydrolyse 1,6-a-D-glucosidic branchpoints.
Commercial enzymes used for the industrial hydrolysis of starch are produced by
Bacillus amyloliquefaciens (supplied by various manufacturers) and by B.
licheniformis (supplied by Novo Industri A/S as Termamyl). They differ
principally in their tolerance of high temperatures, Termamyl retaining more
activity at up to 110°C, in the presence of starch, than the B.
amyloliquefaciens a-amylase. The maximum DE obtainable using bacterial a-amylases
is around 40 but prolonged treatment leads to the formation of maltulose (4-a-D-glucopyranosyl-D-fructose),
which is resistant to hydrolysis by glucoamylase and a-amylases. DE values of
8-12 are used in most commercial processes where further saccharification is to
occur. The principal requirement for liquefaction to this extent is to reduce
the viscosity of the gelatinised starch to ease subsequent processing.
Various
manufacturers use different approaches to starch liquefaction using a-amylases
but the principles are the same. Granular starch is slurried at 30-40% (w/w)
with cold water, at pH 6.0-6.5, containing 20-80 ppm Ca2+ (which stabilises and
activates the enzyme) and the enzyme is added (via a metering pump). The a-amylase
is usually supplied at high activities so that the enzyme dose is 0.5-0.6 kg
tonne-1 (about 1500 U kg-1 dry matter) of starch. When Termamyl is used, the
slurry of starch plus enzyme is pumped continuously through a jet cooker, which
is heated to 105°C using live steam. Gelatinisation occurs very rapidly and the
enzymic activity, combined with the significant shear forces, begins the
hydrolysis. The residence time in the jet cooker is very brief. The partly
gelatinised starch is passed into a series of holding tubes maintained at
100-105°C and held for 5 min to complete the gelatinisation process. Hydrolysis
to the required DE is completed in holding tanks at 90-100°C for 1 to 2 h. These
tanks contain baffles to discourage backmixing. Similar processes may be used
with B. amyloliquefaciens a-amylase but the maximum temperature of 95°C must not
be exceeded. This has the drawback that a final 'cooking' stage must be
introduced when the required DE has been attained in order to gelatinise the
recalcitrant starch grains present in some types of starch which would otherwise
cause cloudiness in solutions of the final product.
The liquefied
starch is usually saccharified but comparatively small amounts are spray-dried
for sale as 'maltodextrins' to the food industry mainly for use as bulking
agents and in baby food. In this case, residual enzymic activity may be
destroyed by lowering the pH towards the end of the heating period.
Fungal a-amylase
also finds use in the baking industry. It often needs to be added to bread-making
flours to promote adequate gas production and starch modification during
fermentation. This has become necessary since the introduction of combine
harvesters. They reduce the time between cutting and threshing of the wheat,
which previously was sufficient to allow a limited sprouting so increasing the
amounts of endogenous enzymes. The fungal enzymes are used rather than those
from bacteria as their action is easier to control due to their relative heat
lability, denaturing rapidly during baking.