The Mechanism of Enzyme Action
In order for a reaction to occur, reactant molecules must contain sufficient
energy to cross a potential energy barrier, the activation energy. All
molecules possess varying amounts of energy depending, for example, on their
recent collision history but, generally, only a few have sufficient energy for
reaction. The lower the potential energy barrier to reaction, the more reactants
have sufficient energy and, hence, the faster the reaction will occur. All
catalysts, including enzymes, function by forming a transition state, with the
reactants, of lower free energy than would be found in the uncatalysed reaction
(Figure 1.1). Even quite modest reductions in this potential energy barrier may
produce large increases in the rate of reaction (e.g. the activation energy for
the uncatalysed breakdown of hydrogen peroxide to oxygen and water is 76 kJ M-1
whereas, in the presence of the enzyme catalase, this is reduced to 30 kJ M-1
and the rate of reaction is increased by a factor of 108, sufficient
to convert a reaction time measured in years into one measured in seconds).

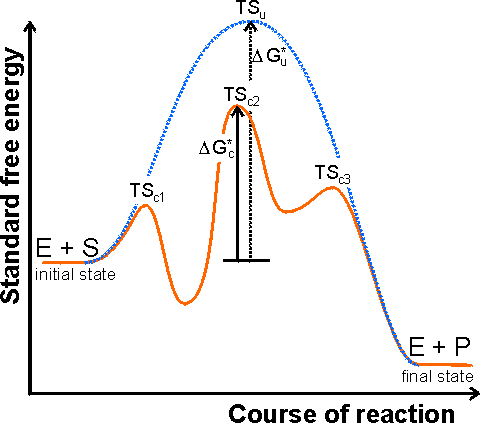
Figure 1.1. A schematic diagram showing the free energy profile of the course of an enzyme catalysed reaction involving the formation of enzyme-substrate (ES) and enzyme-product (EP) complexes, i.e.
![]()
The catalysed reaction pathway goes through the transition states TSc1, TSc2 and TSc3, with standard free energy of activation DGc*, whereas the uncatalysed reaction goes through the transition state TSu with standard free energy of activation DGu*. In this example the rate limiting step would be the conversion of ES into EP. Reactions involving several substrates and products, or more intermediates, are even more complicated. The Michaelis-Menten reaction scheme [1.7] would give a similar profile but without the EP-complex free energy trough. The schematic profile for the uncatalysed reaction is shown as the dashed line. It should be noted that the catalytic effect only concerns the lowering of the standard free energy of activation from DGu* to DGc* and has no effect on the overall free energy change (i.e.. the difference between the initial and final states) or the related equilibrium constant.
There are a number of mechanisms by which this activation energy decrease may be achieved. The most important of these involves the enzyme initially binding the substrate(s), in the correct orientation to react, close to the catalytic groups on the active enzyme complex and any other substrates. In this way the binding energy is used partially in order to reduce the contribution of the considerable activation entropy, due to the loss of the reactants' (and catalytic groups') translational and rotational entropy, towards the total activation energy. Other contributing factors are the introduction of strain into the reactants (allowing more binding energy to be available for the transition state), provision of an alternative reactive pathway and the desolvation of reacting and catalysing ionic groups.
The energies available to enzymes for binding their substrates are determined primarily by the complementarity of structures (i.e. a good 3-dimensional fit plus optimal non-covalent ionic and/or hydrogen bonding forces). The specificity depends upon minimal steric repulsion, the absence of unsolvated or unpaired charges, and the presence of sufficient hydrogen bonds. These binding energies are capable of being quite large. As examples, antibody-antigen dissociation constants are characteristically near 10-8 M (free energy of binding is 46 kJ M-1), ATP binds to myosin with a dissociation constant of 10-13 M (free energy of binding is 75 kJ M-1) and biotin binds to avidin, a protein found in egg white, with a dissociation constant of 10-15 M (free energy of binding is 86 kJ M-1). However, enzymes do not use this potential binding energy simply in order to bind the substrate(s) and form stable long-lasting complexes. If this were to be the case, the formation of the transition state between ES and EP would involve an extremely large free energy change due to the breaking of these strong binding forces, and the rate of formation of products would be very slow. They must use this binding energy for reducing the free energy of the transition state. This is generally achieved by increasing the binding to the transition state rather than the reactants and, in the process, introducing an energetic strain into the system and allowing more favourable interactions between the enzyme's catalytic groups and the reactants.