[1.1]
All enzymes contain a protein backbone. In some enzymes this is the only
component in the structure. However there are additional non-protein moieties
usually present which may or may not participate in the catalytic activity of
the enzyme. Covalently attached carbohydrate groups are commonly encountered
structural features which often have no direct bearing on the catalytic activity,
although they may well effect an enzyme's stability and solubility. Other
factors often found are metal ions (cofactors) and low molecular weight
organic molecules (coenzymes). These may be loosely or tightly bound by
noncovalent or covalent forces. They are often important constituents
contributing to both the activity and stability of the enzymes. This requirement
for cofactors and coenzymes must be recognised if the enzymes are to be used
efficiently and is particularly relevant in continuous processes where there may
be a tendency for them to become separated from an enzyme's protein moiety.
Enzymes are classified according the report of a Nomenclature Committee appointed by the International Union of Biochemistry (1984). This enzyme commission assigned each enzyme a recommended name and a 4-part distinguishing number. It should be appreciated that some alternative names remain in such common usage that they will be used, where appropriate, in this text. The enzyme commission (EC) numbers divide enzymes into six main groups according to the type of reaction catalysed:
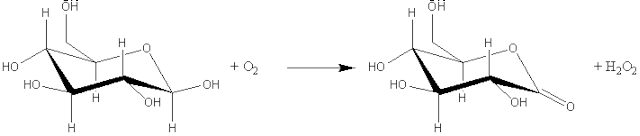
(1) Oxidoreductases which involve redox reactions in which hydrogen or oxygen atoms or electrons are transferred between molecules. This extensive class includes the dehydrogenases (hydride transfer), oxidases (electron transfer to molecular oxygen), oxygenases (oxygen transfer from molecular oxygen) and peroxidases (electron transfer to peroxide). For example: glucose oxidase (EC 1.1.3.4, systematic name, b-D-glucose:oxygen 1-oxidoreductase).

[1.1]
b-D-glucose +
oxygen ![]() D-glucono-1,5-lactone +
hydrogen peroxide
D-glucono-1,5-lactone +
hydrogen peroxide
(2) Transferases which catalyse the transfer of an atom or group of atoms (e.g. acyl-, alkyl- and glycosyl-), between two molecules, but excluding such transfers as are classified in the other groups (e.g. oxidoreductases and hydrolases). For example: aspartate aminotransferase (EC 2.6.1.1, systematic name, L-aspartate:2-oxoglutarate aminotransferase; also called glutamic-oxaloacetic transaminase or simply GOT).
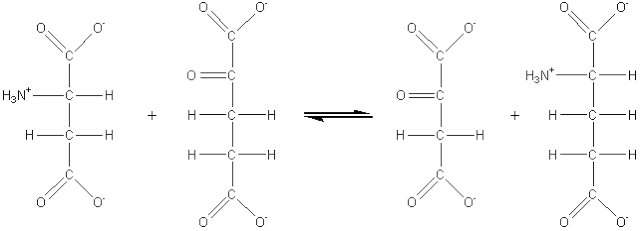
[1.2]
L-aspartate + 2-oxoglutarate
![]() oxaloacetate + L-glutamate
oxaloacetate + L-glutamate
(3) Hydrolases which involve hydrolytic reactions and their reversal. This is presently the most commonly encountered class of enzymes within the field of enzyme technology and includes the esterases, glycosidases, lipases and proteases. For example: chymosin (EC 3.4.23.4, no systematic name declared; also called rennin).
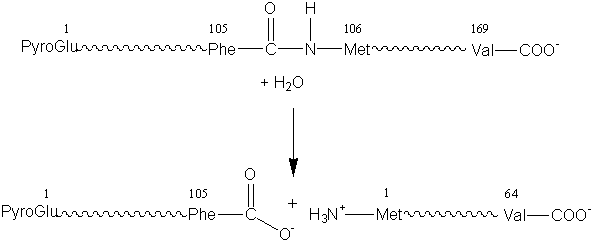
[1.3]
k-casein + water
![]() para-k-casein
+ caseino macropeptide
para-k-casein
+ caseino macropeptide
(4) Lyases which involve elimination reactions in which a group of atoms is removed from the substrate. This includes the aldolases, decarboxylases, dehydratases and some pectinases but does not include hydrolases. For example: histidine ammonia-lyase (EC 4.3.1.3, systematic name, L-histidine ammonia-lyase; also called histidase).
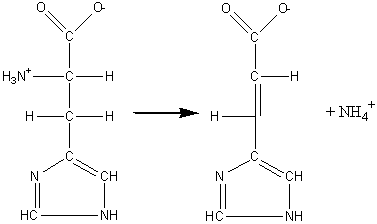
[1.4]
L-histidine
![]() urocanate + ammonia
urocanate + ammonia
(5) Isomerases which catalyse molecular isomerisations and includes the epimerases, racemases and intramolecular transferases. For example: xylose isomerase (EC 5.3.1.5, systematic name, D-xylose ketol-isomerase; commonly called glucose isomerase).
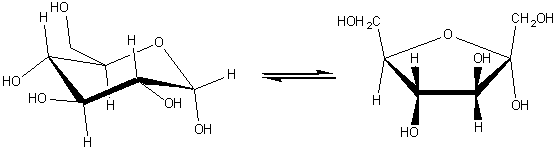 [1.5]
[1.5]
a-D-glucopyranose
![]() a-D-fructofuranose
a-D-fructofuranose
(6) Ligases, also known as synthetases, form a relatively small group of enzymes which involve the formation of a covalent bond joining two molecules together, coupled with the hydrolysis of a nucleoside triphosphate. For example: glutathione synthase (EC 6.3.2.3, systematic name, g-L-glutamyl-L-cysteine:glycine ligase (ADP-forming); also called glutathione synthetase).
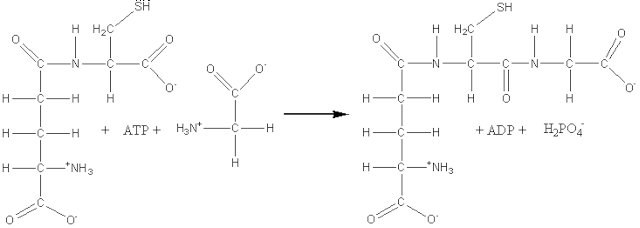
[1.6]
ATP + g-L-glutamyl-L-cysteine
+ glycine ![]() ADP + phosphate +
glutathione
ADP + phosphate +
glutathione